Plasma Technology
Adtec joins the European Research Association for Thin Films
We are pleased to announce our membership to the European Research Association for Thin Films.
The EFDS is a fantastic community of renowned specialists within the European Thin-Film, Semiconductor and Vacuum Technology industries.
We are hugely excited for the opportunity to learn from industry experts and collaborate with new partners

Dedicated Adtec RF and Cold Plasma News
Hello and welcome!
As part of our brand new website, we are excited to launch a fresh news feed, dedicated to our RF and Cold Plasma activities.
Here, we will discuss products, innovations, applications and publications - we have a lot in the pipeline for 2021, and we can't wait to share it all with you!
Our medical division, Adtec Healthcare, can now be found here. You can still find all the old posts in the archives of our news section.
Happy New Year from all of us here at Adtec,
See you soon!
New Actinic Keratoses clinical trial publication release

Congratulations Professor Alexander Rösch and his team at the Department of Dermatology at the University Hospital Essen for their recent clinical trial publication. The study “Efficacy of cold atmospheric plasma versus diclofenac 3% gel in patients with actinic keratoses: a prospective, randomized, and rater-blinded study (ACTICAP)” can be found here: https://onlinelibrary.wiley.com/doi/epdf/10.1111/jdv.16735
Their clinical trial began in 2017 and its purpose was to test the efficacy of our medical device, the Adtec SteriPlas on actinic keratoses patients in comparison to those treated with Diclofenac 3% gel. The clinical efficacy of this modality is comparable to that of Diclofenac gel. However, unlike Diclofenac, cold atmospheric plasma showed no major side effects. Thus, this modality might be especially well-suited for patients who require nontoxic treatment options, particularly immunocompromised patients and those with extensive field cancerization. This continues to be one of our strongest and most favoured characteristics of the Adtec SteriPlas: no side effects.
We encourage you to read the clinical trial publication and welcome any questions that you have.
#actinickeratoses #actinickeratosis #skin #dermatology #coldplasma #kaltesplasma #clinicaltrial #sideeffects
Adtec Healthcare's new project with Hull York Medical School testing gas plasma on Osteomyelitis bone infections.
We are excited to announce the study “Gas Plasma for the Prevention and Management of Osteomyelitis Biofilms” has now begun at the Hull York Medical School. The study led by Dr Angela Oates and funded by the National Biofilms Innovation Centre will seek to test the efficacy of our gas plasma system on osteomyelitis bone infections using our PlasmaTact device. This project will develop a novel laboratory testing model to evaluate and optimise plasma treatment for osteomyelitis biofilm infections.

We have already demonstrated that our gas plasma medical device, the Adtec SteriPlas, has proven antibacterial efficacy and accelerated healing in problematic and non-healing wounds with strong evidence collected from our extensive library of clinical trials and publications. This includes diabetic foot ulcers which are often complicated by biofilm. Over the course of its existence it has shown that no side effects have been reported which offers patients a safe and reliable treatment option. It has also shown that a 2-minute treatment time is sufficient to achieve exceptional results and that it is also a broad spectrum antibacterial with the ability to kill a wide range of Gram-negative and Gram-positive bacterial superinfections.
The PlasmaTact utilizes the same gas plasma technology from the Adtec SteriPlas. The main difference between the two devices is that the PlasmaTact offers a smaller handheld style treatment area of 1cm2 (vs 12cm2 with the SteriPlas) and offers users the ability to change the power output settings (vs the fixed settings with the SteriPlas).
Osteomyelitis (OM) is biofilm infection of the bone and is a common and costly complication in diabetic foot ulcer (DFU) patients often resulting in amputations. Long-term antimicrobial therapy is widely used as a primary treatment for OM or as an adjunct to surgical approaches however, there is a failure rate of up to 35% and an associated increasing prevalence of antimicrobial resistance. There is an urgent clinical need to develop alternative non-antimicrobial approaches to treat infections, and currently there is a lack of credible alternatives in the market for the treatment of OM.
Evaluating the efficacy of gas plasma in OM represents unique challenges as both the biofilm growth and physical characteristics of OM bone will affect the delivery and activity of the plasma. In vitro biofilm model systems offer a means by which rapid, costs effective and standardised evaluation of anti-biofilm treatments can be undertaken.
The PATROL project is a new and exciting collaborative project between Adtec Healthcare and Dr Angela Oates of the University of Hull Wound Healing Group which is supported by NBIC PoC Award. The overall aim of this project is to develop an osteomyelitis biofilm infection model to support the optimisation and evaluation of a cold plasma technology for the management and prevention of OM.
This collaborative project represents the first phase of the novel utilisation of Adtec SteriPlas to treat OM or as an adjunct current therapy. Successful translation of this technology to treat OM will reduce antimicrobial usage and associated OM amputations, reducing NHS costs and ultimately improving patient outcomes.
#osteomyelitis #biofilm #boneinfection #infection #antimicrobial #antimicrobialresistance #amputation #plasma #gasplasma #coldplasma #kaltesplasma #NBIC #universityofhull #podiatry #diabeticfootulcer #diabeticfoot #diabetes #hullyorkmedicalschool
Adtec Healthcare confirms attendance for 3rd conference in 2020
Adtec Healthcare looks forward to exhibiting at the Diabetic Foot Study Group (DFSG) conference in Bratislava, Slovakia between 18-20 September 2020.
You can find us at booth 13 in the exhibition hall where our staff will be ready to welcome you. We will also have our flagship medical device, the Adtec SteriPlas, live on demonstration to show you how easy it is to treat diabetic foot ulcers.

#plasma #coldplasma #kaltesplasma #gasplasma #DFSG #DFSG2020 #diabeticfootulcer #wound #woundcare #woundhealing #wundkongress #wund #medicaldevice #DFSG #DFSG2020
Adtec Healthcare exhibiting at two leading wound conferences this year
Adtec Healthcare looks forward to exhibit at the European Wound Management Association (EWMA) conference and The Malvern Diabetic Foot Conference in May 2020. As the two conferences will be running on the same dates (13th-15th May ’20), we will have teams exhibiting at both conferences so we can meet with you at whichever conference you attend.
We will have our medical device live on display at both exhibitions and look forward to inviting you to our booth to give a demonstration on its ease of use to achieve remarkable results.


#EWMA #EWMA2020 #MalvernDFU #Malvern2020 #wound #wundkongress #coldplasma #kaltesplasma #gasplasma #plasma #exhibition #woundcare #surgicalsiteinfection #preventSSI #SSIprevention
Can two plasma medical devices have the same clinical efficacy?
We have been asked by Health Care Professionals to clarify , “What is the difference between the Adtec SteriPlas (Adtec Healthcare) and other cold plasma technologies such as Plasma Care® ?” or “Can two different plasma technologies have the same clinical efficacy… what’s the difference?”.
The answers to these questions are that there is a significant difference between plasma technologies, and it is therefore not possible to claim a similarity in clinical efficacy as the plasma treatments would be different.
Two devices do not produce the same plasma and it is the composition of the plasma that exerts the effects, beneficial and detrimental. For the importance of patient safety, Adtec Healthcare advises against any other plasma device claiming clinical efficacy from the clinical evidence of the CE approved SteriPlas gas plasma. Adtec’s clinical data should never be used to compare to any other plasma treatment which have not yet been clinically tested.
Atmospheric pressure Plasma has a myriad of potential medical applications from low energy plasmas used for wound healing to higher energy plasmas used to cut bones during an operation or for coagulation. The main differences in all plasma technologies include the different forms of plasmas electrode source designs, types of energy used (RF, DC and microwave) and gas used (argon, helium or nitrogen etc) versus air. The Adtec SteriPlas (MicroPlaSter) plasma technology is based on a microwave powered plasma jet utilising argon gas and the other technology is a DC powered Surface Micro Discharge (SMD) plasma source utilising air as carrier gas. The type of plasma generated corresponds directly to the type of treatment delivered to the patient. Each type of plasma delivers a specific type of treatment.
During the pre-clinical trials and studies, Max Planck had conducted a study illustrating the noticeable differences of our argon gas microwave plasma treatment vs the surface micro-discharge (SMD) ‘Air plasma’ treatment developed at MPI. In this study, clear distinctions could be observed such as argon gas plasma treatment included predictable, safe and low dosages of reactive species whereas air-based plasmas would deliver significantly higher concentrations (over 37 times more than argon microwave plasma). Air Plasma has much higher levels of NOx and Ozone than argon gas plasma and needs to be tested not only clinically but also safety for the operator. It is essential to verify the product safety and clinical safety of these higher levels of NOx. The air-based plasmas are also generally dependent on the surrounding environment; temperature and humidity will affect the way the plasmas are generated and therefore difficult to deliver the same treatment each time the plasma is generated.

Adtec Plasma Technology has been at the forefront for the development of plasma products for over 30 years, proudly placing us as one of the leaders in the semiconductor and RF plasma market. Adtec has also developed other plasma technologies (gas and air) for remote plasma, gas abatement and surface treatment applications.
In 2002, we designed our first cold plasma technology showing painless effect on contact with human skin. This revolutionised the way how we are used to dealing with plasmas – typically sought as too hot to touch but now redesigned to be colder and harmless on contact with skin. In 2004, our research results were presented at a plasma conference showing distinctive microbial load reduction was possible when bacteria were exposed to our gas plasma.
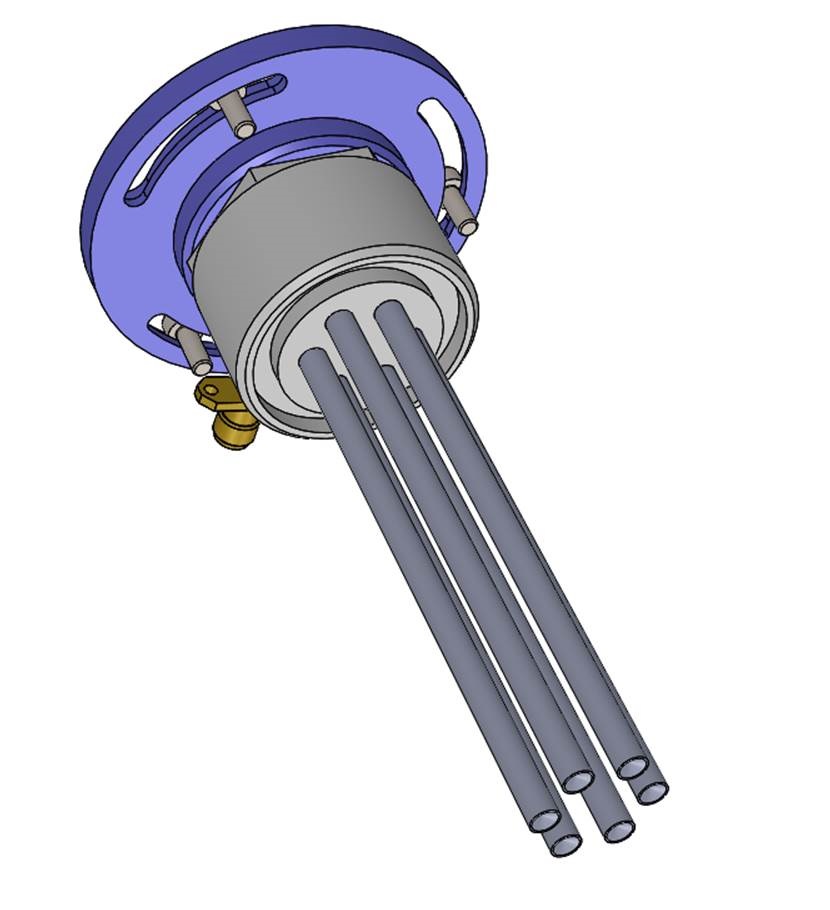
Later in 2004, Adtec introduced this plasma technology to the Max Planck Institute for Extraterrestrial Physics leading to a collaboration in plasma medicine. The Adtec plasma source was adapted to a wide area plasma source in collaboration with MPI. This plasma source of the medical device is a shared patent with Adtec and MPI (as seen in the image below). This plasma source is one of the critical components used in the SteriPlas and MicroPlaSter. Adtec solely and exclusively designed and developed the medical device prototypes and products including customized components, electrical, mechanical and software program satisfying the strict standards of European medical device regulations.


We are grateful to the plasma medicine team at Max Planck for having an interest in our plasma technology system, for carrying out extensive scientific research and for managing the clinical trials using the Adtec MicroPlaSter. These clinical trials placed our medical device as the first worldwide to be used in clinical trials on wounds, paving a new treatment programme that would later be adopted by other companies with an interest to develop gas plasma medical devices. The extensive research and clinical testing assure us of the safety of the product and the technology.
The new European MDR regulations coming into force next year also require all companies to produce proper clinical evidence to support their product claims. We do emphasize the importance of proper pre-clinical and clinical testing of all types of plasma technologies in order to be assured of the product and clinical safety. Adtec Healthcare do encourage and support plasma companies in this industry as our goal is to make gas plasma a recognised treatment option for patients with wounds, surgical site infections and dermatological conditions.
Growing media coverage of Adtec's plasma research project with Cranfield University
We’re excited to see growing media coverage of our recent post on our plasma research project with Cranfield University.
You can see the article on @CranfieldUni ‘s page: https://www.cranfield.ac.uk/press/news-2019/students-research-cleaning-solar-collectors-without-water#.XREueOGrOlc.email
It can also be found on @Solar_And_Power’s page: https://solarpowermanagement.net/article/107599/Students_Research_Cleaning_Solar_Collectors_Without_Water
And @NACleanEnergy ‘s page: http://www.nacleanenergy.com/articles/34831/students-research-cleaning-solar-collectors-without-water
The focus of this research is to understand the utilisation of plasma-assisted surface conditioning of low-iron glass solar reflecting mirrors for concentrating solar thermal power applications. The research project is supervised by leading experts in this field Professor Chris Sansom and Dr Peter King of Cranfield University and Dr Adam Bennett of Cranfield Plasma Solutions.
Currently much water is used to clean the mirrors, a precious resource in arid terrains. The aim of this project is to investigate the characteristics of a novel atmospheric pressure plasma system used to condition CSP concentrating mirrors which will be capable of reducing the amount of water used in the cleaning process.
#CSP #concentratingsolarpower #solarpower #solar #solarenergy #renewableenergy #greenenergy #energy #cleanenergy #sustainableenergy #sustainablepower #sustainableenvironment #gasplasma #airplasma
Adtec Plasma in CSP Renewable Energy Project

Adtec Europe Ltd is sponsoring two post-graduate students to do research in new potential applications for plasma in collaboration with Cranfield University.
The focus of this research is to understand the utilisation of plasma-assisted surface conditioning of low-iron glass solar reflecting mirrors for concentrating solar thermal power applications. The research project is supervised by leading experts in this field Professor Chris Sansom and Dr Peter King of Cranfield University and Dr Adam Bennett of Cranfield Plasma Solutions.
CSP plants generate electricity by concentrating sun light with large arrays of mirrors which are usually located in desert regions. Consequently, the mirrors get covered in sand and dust, and require cleaning with brushes and water on a regular basis. Currently much water is used to clean the mirrors, a precious resource in arid terrains. The aim of this project is to investigate the characteristics of a novel atmospheric pressure plasma system used to condition CSP concentrating mirrors which will be capable of reducing the amount of water used in the cleaning process.
The Global Concentrating Solar Power (CSP) market was valued at over $3 Billion US in 2016 and is anticipated to grow by 13% by 2025. There is also a compelling business desire to undertake this project. The development of a novel atmospheric pressure plasma system will be a significant game changer in the CSP market. Such a disruptive technology is anticipated to yield significant commercial benefits.

#CSP #concentratingsolarpower #solarpower #solar #solarenergy #renewableenergy #greenenergy #energy #cleanenergy #sustainableenergy #sustainablepower #sustainableenvironment #plasma #gasplasma #coldplasma #kaltesplasma #airplasma
EWMA
We had a great time exhibiting at the EWMA 2019 conference. We would like to congratulate Maurice Moelleken, Dr Heinrich Rotering and Dr Michael Pierides for their brilliant presentations during the conference. Their data showed the strong benefits of using the Adtec SteriPlas on chronic wounds and surgical site infections, offering an alternative to standard treatment therapies that bacteria may pose a resistance to.
The Adtec SteriPlas has proven antibacterial efficacy backed by a wide clinical bibliography and no side effects reported making it safe, painless and effective for the treatment of infected wounds stalled by bacteria.
For more information send us an email to info@adtec.eu.com


#EWMA #EWMA2019 #antimicrobialresistance #medicaldevice
#gasplasma #coldplasma #kaltesplasma