cold plasma
PlasmaTact for Surface Energy Modification and Bonding on Aerospace Composites
Introduction
The aerospace sector is pushing towards sustainability and reduction in their carbon emissions, as a result, there is a demand for lighter and more fuel-efficient aircraft. ‘Light weighting’ can be achieved using fibre-reinforced composite materials to construct primary and secondary structures of an aircraft. Circa 50% of the mass of the Boeing 787 ‘Dreamliner’ is accounted for by composite materials. Advantages such as excellent fatigue life, corrosion resistance, and improved thermal and electrical properties coupled with the weight-saving capability make fibre-reinforced composite materials desirable to aircraft manufacturers.
![Usage of composites in Boeing 787 Dreamliner structure [22].](https://www.researchgate.net/publication/320951033/figure/fig1/AS:631662626996225@1527611514245/Usage-of-composites-in-Boeing-787-Dreamliner-structure-22.png)
The most common surface treatments are techniques such as sanding or grit blasting that physically abrade the bond surface, increasing the surface roughness that aids in mechanical interlocking between the adhesive and the adherend. These preparation techniques produce dust, which is undesirable for bonding, so solvent cleaning is usually performed after sanding. Plasma treatments are gaining widespread application in the automotive and aerospace industry as they not only clean the surface, but also change the chemical composition and surface energy aiding in an improved bonded joint.
Plasma treatments are gaining widespread application in the automotive and aerospace industry as they not only clean the surface, but also change the chemical composition and surface energy aiding in an improved bonded joint.
PlasmaTact 50 Testing
The main effects of plasma treatment on a composite surface are: modifying surface roughness due to the ablation effect; removal of contaminants; and, altering the surface chemistry. Hence, treatment of the composite adherends using plasma may activate both the mechanical interlocking and the chemical bonding adhesion mechanisms, maximising the adhesive bond strength. Concerning surface chemistry, research has found that it generally improves the oxygen content and creates polar components on the surface, which are essential for adhesive bonding [v]. Due to all these advantages,plasma treatment is gaining widespread applications in the aerospace and automotive sectors for use in adhesive bonding, with one of the most used plasma treatment systems being Atmospheric Pressure Plasma (APP) systems, due to their low operating costs and ease of use.
Surface Treatments
All composite samples were cleaned in an ultrasonic bath for 20 minutes before treatment. One set of samples was treated and characterised. For solvent cleaning, Isopropanol was used. For sanding, 240-grit sandpaper was used to abrade the surface in random directions; the grit size and sanding directions were chosen based on literature. The plasma torch parameters were set so that the optimal plasma was discharged for surface energy modification using pure argon gas: 50 W forward power, 0 W reflected power, 2.48 GHz frequency, 10 L/min gas flow rate, a 3 mm stand-off distance, and a processing speed of 1m/min.

Contact Angle (lower is better)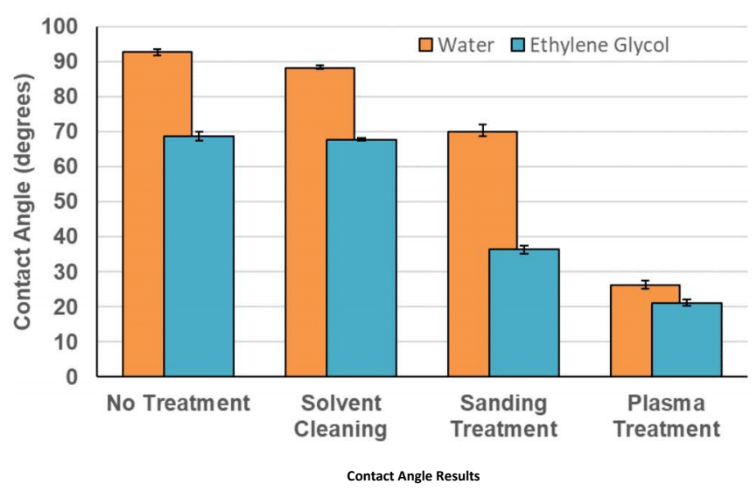
The non-treated sample has a hydrophobic surface, with a water contact angle of 92.1° and an ethylene glycol contact angle of 68.7°. Ethylene glycol contact angle will always be lower than water, on this material, as it has lesser surface tension. The solvent-cleaned sample has a marginally reduced contact angle; however, the sanding-treated samples have significantly reduced contact angles of 70.0°and 36.3° for water and ethylene glycol, respectively. Plasma treatment has a bigger reduction in contact angle: 26.1° and 21.1°, signifying a highly energised, hydrophilic surface.
Surface Energy (Higher is better)
The contact angles in Figure 10 were then used with the Wendt, Rabel and Kaelble method, to determine the surface energy of each composite sample. Figure above shows the results. The plasma
treated surface has the highest surface energy of 74.5mJ/m2, followed by the sanding-treated samples with a value of 37.1mJ/m2. The non-treated and solvent-cleaned samples have low surface energies of 20.7mJ/m2 and 22.0mJ/m2, respectively.
Surface Roughness (Lower is better)
The non-treated, solvent cleaned, and plasma treated samples have low roughness values of 0.16μm, 0.18μm, and 0.18μm, Ra, respectively. The sanding treatment has the highest Ra value of 0.64μm, because of the physical abrasion from the sandpaper.
Lap Shear Failure Load (Higher is better)
The average failure loads of the hybrid plasma treatment were lower than the plasma treated only specimens, which is an important outcome. Plasma treatment alone, was better than using any other treatment and better than using a hybrid approach with the next best treatment method.
Conclusion
The 50W Plasma Tact from Adtec Plasma improved the bonding strength of a carbon fibre composite. The plasma treatment may also be used, on it’s own, without the need for using traditional solvents or sand blasting techniques.
For more information regarding the PlasmaTact Series, click here.
Cold Atmospheric Plasma: Enhancing EV Battery Manufacturing & Performance
As Electric Vehicles become a part of everyday life, manufacturers are required to continually improve battery technology and performance: more energy storage, operating at higher energy densities. Cold Atmospheric Plasma is a pioneering technology that offers a novel solution. By introducing plasma treatments into existing manufacturing processes, it is possible to improve physical bonding of components, increase electrical conductivity of batteries, and increase thermal diffusivity across components.
The Manufacturing Challenge
The future of propulsion systems is electric drivetrains, often powered by electrical energy stored in batteries. With electric cars now commonplace, we are starting to other EVs enter the market, including motorbikes, yachts and light aircraft. To be effective and efficient, batteries for electric vehicles must exhibit good temperature management, be weather resistant, and comprise of a high-quality assembly to ensure a long service life. To achieve these, stable assembly processes that include reliable bonding technologies are essential. The treatment of surfaces using atmospheric plasma technologies is one of the most effective industrial processes for cleaning, activating, or coating plastics, metals, glass and other materials.
The use of plasma to optimise bonding during module assembly processes has the additional benefit of increasing the thermal and electrical conductivity across material junctions. Furthermore, it is a green technology: plasma replaces chemicals, reducing carcinogenic emissions from solvent-containing products.

The Technology Solution
Prismatic cell treatment is performed before isolation, which increases surface energy for improved deposition of isolation paints and activation for cell-to-cell bonding. Between the cells there is heat conductive glue, which needs to remain mechanically strong whilst allowing the cells to thermally cycle. Plasma cleans and changes the micro-topography of the paint surface; this increases the surface area to volume ratio, in turn increasing the rate the cell dissipates thermal energy. Activation is also performed to increase the bonding between prismatic cells and pack bases. A typical pack base material is PolyCarbonate (PC).



Pouch cells have very low mass and high energy density. Originally used in mobile phones, they are now being selected by some leading EV manufactures. Pouch cells require cleaning before laser welding of the electrical connections. Plasma is used to clean and activate the cell surface to improve the bonding with tape or adhesives. Plasma densifies the oxide layer and strengthens it. Organic substances are removed. Plasma deposits free O and OH groups for increased adhesion. When cells charge and discharge, they expand and contract, which is a challenge for adhesion and thermal dissipation: plasma adds value by also improving the thermal diffusivity across component surfaces.
Cylindric cells were originally pioneered by Tesla. The outside top surfaces of the individual cells are bonded to a polymer frame to ensure physical alignment. A thin wire is ultrasonically welded to the top of the cell, then connected to a busbar. If contamination is present on the surface of the cell electrode, the bond to the wire will fail. A plasma clean can be used to prevent this from happening, ensuring components are made right the first time.
Glycol coolant is run through battery packs to extract thermal energy, often carried by aluminium Serpentine cooling tubes. These tubes must remain electrically insulated, so a Mylar polymer is wrapped around them. Polymers can be notoriously tricky to bond, so a plasma pre-treatment improves both this bond, and the bond between each Mylar-covered tube.
Plasma processing, therefore, increases the physical bonding of the components inside a battery pack, increases the electrical conductivity of the batteries, and increases the thermal diffusivity across the component surfaces.
The Science – Processing with the Adtec 50W PlasmaTact
The Adtec PlasmaTact is a microwave induced cold plasma torch that generates argon plasma. By installing the device in a CNC machine, we performed surface energy modification on these key materials to enhance the battery manufacturing process.
Mylar, Polycarbonate and Aluminium 6082 were processed, and a metric called water contact angle was used to quantify the surface modification. A reduced contact angle = increased wettability = improved bonds.

Figure 6 demonstrates how the plasma creates regions of super-hydrophilicity, causing water to stick preferentially to the treated area. The 50W PlasmaTact modifies the surface energy (increased surface energy = increased wettability = improved bonding) of the component surfaces. As well as improved physical bonding, the process improves both electrical bonding and thermal diffusivity across the component surfaces.
To find our more about Adtec's pioneering cold plasma technology, please contact us here.
Adtec launches 50W Cold Atmospheric Plasma Torch
Adtec first started to investigate cold atmospheric plasma back in 2005, as part of an anti-bacterial study in collaboration with the Max Planck Institute in Germany.
From here, we developed our patented coaxial microwave plasma technology, which remains at the core of our cold plasma products to this day.
As well as medical applications, Adtec developed PlasmaTact - a 15W torch for surface treatments and anti-bacterial applications.
We are now proud to announce the new, higher power 50W PlasmaTact. The increased power means that not only can we perform the same surface treatment tasks at higher speed, but a greater breadth of surface engineering applications become a possibility - keep an eye out for news on our upcoming work exploring etching and deposition at atmospheric pressure!
New Actinic Keratoses clinical trial publication release

Congratulations Professor Alexander Rösch and his team at the Department of Dermatology at the University Hospital Essen for their recent clinical trial publication. The study “Efficacy of cold atmospheric plasma versus diclofenac 3% gel in patients with actinic keratoses: a prospective, randomized, and rater-blinded study (ACTICAP)” can be found here: https://onlinelibrary.wiley.com/doi/epdf/10.1111/jdv.16735
Their clinical trial began in 2017 and its purpose was to test the efficacy of our medical device, the Adtec SteriPlas on actinic keratoses patients in comparison to those treated with Diclofenac 3% gel. The clinical efficacy of this modality is comparable to that of Diclofenac gel. However, unlike Diclofenac, cold atmospheric plasma showed no major side effects. Thus, this modality might be especially well-suited for patients who require nontoxic treatment options, particularly immunocompromised patients and those with extensive field cancerization. This continues to be one of our strongest and most favoured characteristics of the Adtec SteriPlas: no side effects.
We encourage you to read the clinical trial publication and welcome any questions that you have.
#actinickeratoses #actinickeratosis #skin #dermatology #coldplasma #kaltesplasma #clinicaltrial #sideeffects
Adtec Healthcare's new project with Hull York Medical School testing gas plasma on Osteomyelitis bone infections.
We are excited to announce the study “Gas Plasma for the Prevention and Management of Osteomyelitis Biofilms” has now begun at the Hull York Medical School. The study led by Dr Angela Oates and funded by the National Biofilms Innovation Centre will seek to test the efficacy of our gas plasma system on osteomyelitis bone infections using our PlasmaTact device. This project will develop a novel laboratory testing model to evaluate and optimise plasma treatment for osteomyelitis biofilm infections.

We have already demonstrated that our gas plasma medical device, the Adtec SteriPlas, has proven antibacterial efficacy and accelerated healing in problematic and non-healing wounds with strong evidence collected from our extensive library of clinical trials and publications. This includes diabetic foot ulcers which are often complicated by biofilm. Over the course of its existence it has shown that no side effects have been reported which offers patients a safe and reliable treatment option. It has also shown that a 2-minute treatment time is sufficient to achieve exceptional results and that it is also a broad spectrum antibacterial with the ability to kill a wide range of Gram-negative and Gram-positive bacterial superinfections.
The PlasmaTact utilizes the same gas plasma technology from the Adtec SteriPlas. The main difference between the two devices is that the PlasmaTact offers a smaller handheld style treatment area of 1cm2 (vs 12cm2 with the SteriPlas) and offers users the ability to change the power output settings (vs the fixed settings with the SteriPlas).
Osteomyelitis (OM) is biofilm infection of the bone and is a common and costly complication in diabetic foot ulcer (DFU) patients often resulting in amputations. Long-term antimicrobial therapy is widely used as a primary treatment for OM or as an adjunct to surgical approaches however, there is a failure rate of up to 35% and an associated increasing prevalence of antimicrobial resistance. There is an urgent clinical need to develop alternative non-antimicrobial approaches to treat infections, and currently there is a lack of credible alternatives in the market for the treatment of OM.
Evaluating the efficacy of gas plasma in OM represents unique challenges as both the biofilm growth and physical characteristics of OM bone will affect the delivery and activity of the plasma. In vitro biofilm model systems offer a means by which rapid, costs effective and standardised evaluation of anti-biofilm treatments can be undertaken.
The PATROL project is a new and exciting collaborative project between Adtec Healthcare and Dr Angela Oates of the University of Hull Wound Healing Group which is supported by NBIC PoC Award. The overall aim of this project is to develop an osteomyelitis biofilm infection model to support the optimisation and evaluation of a cold plasma technology for the management and prevention of OM.
This collaborative project represents the first phase of the novel utilisation of Adtec SteriPlas to treat OM or as an adjunct current therapy. Successful translation of this technology to treat OM will reduce antimicrobial usage and associated OM amputations, reducing NHS costs and ultimately improving patient outcomes.
#osteomyelitis #biofilm #boneinfection #infection #antimicrobial #antimicrobialresistance #amputation #plasma #gasplasma #coldplasma #kaltesplasma #NBIC #universityofhull #podiatry #diabeticfootulcer #diabeticfoot #diabetes #hullyorkmedicalschool
Adtec Healthcare confirms attendance for 3rd conference in 2020
Adtec Healthcare looks forward to exhibiting at the Diabetic Foot Study Group (DFSG) conference in Bratislava, Slovakia between 18-20 September 2020.
You can find us at booth 13 in the exhibition hall where our staff will be ready to welcome you. We will also have our flagship medical device, the Adtec SteriPlas, live on demonstration to show you how easy it is to treat diabetic foot ulcers.

#plasma #coldplasma #kaltesplasma #gasplasma #DFSG #DFSG2020 #diabeticfootulcer #wound #woundcare #woundhealing #wundkongress #wund #medicaldevice #DFSG #DFSG2020
Adtec Healthcare exhibiting at two leading wound conferences this year
Adtec Healthcare looks forward to exhibit at the European Wound Management Association (EWMA) conference and The Malvern Diabetic Foot Conference in May 2020. As the two conferences will be running on the same dates (13th-15th May ’20), we will have teams exhibiting at both conferences so we can meet with you at whichever conference you attend.
We will have our medical device live on display at both exhibitions and look forward to inviting you to our booth to give a demonstration on its ease of use to achieve remarkable results.


#EWMA #EWMA2020 #MalvernDFU #Malvern2020 #wound #wundkongress #coldplasma #kaltesplasma #gasplasma #plasma #exhibition #woundcare #surgicalsiteinfection #preventSSI #SSIprevention
EWMA
We had a great time exhibiting at the EWMA 2019 conference. We would like to congratulate Maurice Moelleken, Dr Heinrich Rotering and Dr Michael Pierides for their brilliant presentations during the conference. Their data showed the strong benefits of using the Adtec SteriPlas on chronic wounds and surgical site infections, offering an alternative to standard treatment therapies that bacteria may pose a resistance to.
The Adtec SteriPlas has proven antibacterial efficacy backed by a wide clinical bibliography and no side effects reported making it safe, painless and effective for the treatment of infected wounds stalled by bacteria.
For more information send us an email to info@adtec.eu.com


#EWMA #EWMA2019 #antimicrobialresistance #medicaldevice
#gasplasma #coldplasma #kaltesplasma
Poster presentation at the International Symposium on the Diabetic Foot Conference, Hague.
Wednesday 22nd May 2019
A great poster presentation from Dr Aye Aye Thant at the ISDF 2019 conference!
Dr Thant’s presentation demonstrated the benefits of using the Adtec SteriPlas on diabetic foot ulcer patients.
Prior to the use of the Adtec SteriPlas, all patients in their retrospective study suffered from chronic and non-healing foot and leg ulcers with multi resistant bacteria and recalcitrance to antibiotics. All patients had at least 3 courses of intravenous antibiotics for more than 6 weeks prior with poor response.

The Adtec SteriPlas was then introduced as a treatment option. Instantly, positive results could be seen with the infection management for these patients. For example, one patient who was previously considered for an amputation was now improved and risk free. Healthy granulation could be observed with all patients, offering hope and an alternative to antibiotics that would usually be offered.
For more information on the Adtec SteriPlas, visit our website www.adtecplasma.com or send us an email at info@adtec.eu.com

Mr Keith Cutting presents significant results on DFUs in Vienna conference

Mr Keith Cutting presented at The European Conference on Controversies in Diabetic Foot Management conference in Vienna last week. His presentation focused on the benefits of treating complex diabetic foot ulcer patients with the Adtec SteriPlas.

The Adtec SteriPlas has shown promising results leading to healing in problematic and non-healing wounds such as diabetic foot ulcers or surgical site infections. These complex wounds that have shown signs of stalled healing respond well to intervention with the Adtec SteriPlas.
To learn more about our medical device and the benefits it offers to patients with wounds, surgical site infections and dermatological conditions send us an email at info@adtec.eu.com






